Product Description
-
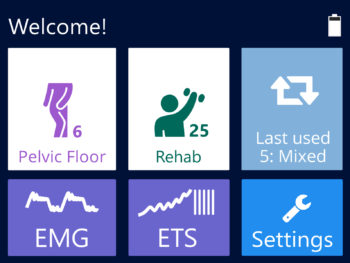
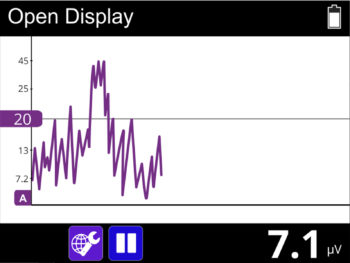
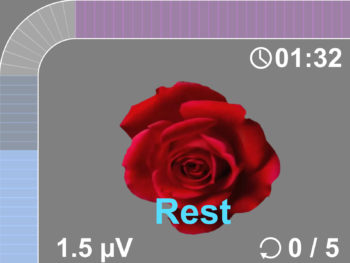
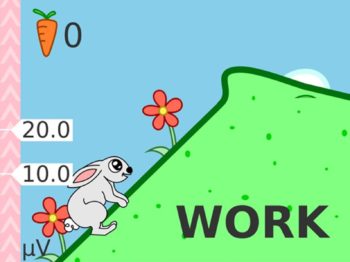
- The new user friendly touch screen based interface makes it a self-explanatory unit with interactive learning on the go, including electrode placement, running EMG graph, EMG biofeedback Games and Templates as well as many other helpful tools.
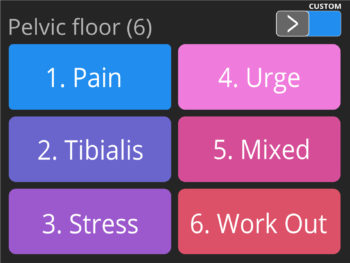
- Pre-set programmes for Incontinence treatment (including a special programme for the treatment of incontinence by stimulating the nervous tibialis posterior with self-adhesive electrodes) and neuro-muscular Rehabilitation.
- Supplied with Rubber sleeve with stand and lanyard attachment option.
- Designed for Physiotherapist in mind to be used as a portable tool. Suitable as a personal trainer for in-clinic or home use (under guidance).
- Single channel EMG (Biofeedback) combined with 1 channel of NMES (stimulation).
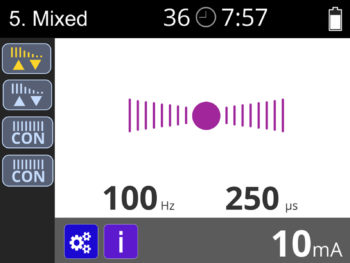
- Single channel ETS (EMG triggered stimulation) with single channel stimulation.
- Simple single-phase or multiphase operation: multiphase allows EMG/ETS/STIM to be combined in one programme.
- Multilingual full color display screen and voice prompts for Biofeedback.
- Manual and Automatic threshold control.
- EMG Biofeedback between stimulation (muscle activity and tiredness indication when using muscle stimulation).
- Stand alone device or used in conjunction with established multilingual software.
- Up to 10 meters of wireless connection for NeuroTrac Software.
- For home users: Produces and prints (with the optional PC Software) the comprehensive patient progress reports on a day to day basis providing average readings on work/rest, peak, onset and muscle release times, work and rest deviations and the average mA current.
- Used as a learning tool for both patient and clinician with emphasis on improving treatment techniques in the use of EMG and Neuromuscular stimulation.
Model number: MYO120P. This product is available through selected distributors who are able to provide training and support.
-
- 1. EMG
- 1.0 Single channel EMG
- 1.1 EMG Range: 0.2 to 2000 μV RMS (continuous)
- 1.2 Sensitivity: 0.l μV RMS
- 1.3 Accuracy: 4% of μV reading +/-0.3 μV at 200 Hz
- 1.4 Selectable Bandpass filter – 3db Bandwidth,
- a. Wide: 18 Hz +/- 4 Hz to 370 Hz +/- 10% – Reading below 235 microvolts 10 Hz +/-3 Hz to 370 Hz +/- 10% – Reading above 235 microvolts
- b. Narrow: 100 Hz +/- 5% to 370 Hz +/- 10%
- 1.5 Notch filter: 50 Hz (Canada 60Hz) – 33 dbs (0. 1% accuracy)
- 1.6 Common Mode Rejection Ratio: 130 dbs Minimum @ 50 Hz
- 1.7 Work / Rest periods: 2-99 seconds
- 1.8 Number of Trials: 2-99
- 2. NMES, TENS and ETS
- 2.1 Single channel Stimulator
- 2.2 Amplitude: 0-90 mA into 500 Ohm load – actual mA will tend to be less than indicated due to Electrode impedance: at 1000 Ohms load (Electrodes in poor condition) the maximum will be limited to 86 mA, at 1500 Ohms load the maximum will be limited to 65 mA.
- 2.3 Type: Constant current, maximum output voltage 180 Volts +10 / -30 Volts
- 2.4 Waveform: Symmetrical, rectangular, bi-phasic with net zero DC current
- 2.5 Pulse width selection: 50-450 μS in predefined, 50-330μS in custom (10% accuracy)
- 2.6 Pulse rate selection: 2-100 Hz (5% accuracy)
- 2.7 Work / Rest periods: 2-99 seconds
- 2.8 Time 1 – 99 minutes
- 2.9 Ramp up time: 0.1 – 9.9 seconds
- 2.10 Preset and user programmable treatment Programs
- 2.11 Automatic output shut off with detection of open electrode above 10 mA (+/- 2mA).
Battery:
Battery set: 4 x 1.5V, AAA battery.
Low battery indication at 4.2-4.4 volts +/- 0.2 volts, automatic shut off when voltage drops below the low indication. Replace the batteries immediately!
The device goes off automatically when not in use (energy saving): for example when in settings and no key is pressed over 1 minute when in stimulation mode (home screen) and all channels are 0mA.Expected average battery set life [of standard 850 mAh, alkaline]:
14-18 hours in STIM, 28 hours in EMG mode.Expected service life:
5 years. Careful use and maintenance extend the life of the unit over the service life limit.Environmental Conditions for use:
+5 to +40 degrees Centigrade. 15-90% Humidity.Environmental conditions for storage & transport:
-25 to +70 degrees Centigrade. 15-90% Humidity.Physical:
Dimensions: Length 150 mm, Width 89 mm, Depth 35 mm.
Weight: MyoPlus Pro device: 140g (without batteries). - 1. EMG
-
Clinical Benefits:
The intended clinical benefits of the different modes of the NeuroTrac MyoPlus Pro devices are:NMES
- Treatment of stress and urge urinary stress incontinence, with an improvement of symptoms, reduction in leakage, and improvement in bladder control
TENS
- Reduction in pain in patients with pelvic pain
- Treatment of stress and urge urinary stress incontinence, with an improvement of symptoms, reduction in leakage, and improvement in bladder control
EMG
Biofeedback of muscle activity, leading to:
- Improved muscle control, motor recovery, and improvement in muscle strength in patients with stroke and/or paresis
- Reduction in pain in patients with pelvic pain
- Improved muscle control in patients with stress and urge urinary incontinence
- Reduction in symptoms in patients with faecal incontinence
ETS
- Rehabilitation of weak muscles in patients with stroke and/or paresis, with motor recovery and improvement in muscle strength
- Treatment of stress and urge urinary stress incontinence, with an improvement of symptoms, reduction in leakage, and improvement in bladder control
- Reduction in symptoms in patients with faecal incontinence
Clinical Safety:
The clinical risks identified in the clinical data, including the review of clinical literature, PMS, and materiovigilance were:- Pain and discomfort (including pain and discomfort due to high-intensity stimulation)
- Skin irritation (including erythema, rash)
- UTI or vaginal infection
- Worsening of leakage
Warnings:
Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the Member State in which the user and/or patient is established.:- This unit must be used with the guidance of a clinician or therapist. This includes the selection of the type of vaginal probe/skin electrodes and placement of skin electrodes.
- Type BF equipment, Continuous Operation.
- Do not insert lead wires into a mains power supply.
- Do not immerse the unit into water or any other substance.
- The unit is not protected from the ingress of water droplets from a shower of rain if used outside the carrying case.
- Do not use this unit in the presence of a flammable anaesthetic gas mixture and air or with Oxygen or Nitrous Oxide.
- This device is 4 x AAA Batteries operated. If using rechargeable Nickel Metal Hydride batteries, be sure to use a CE-approved battery charger. Never connect this unit directly to a battery charger or to any other mains-powered equipment. We advise not to use Ni-Cad rechargeable batteries. Caution: Do not use lithium batteries unless they comply with IEC60086-4.
- To avoid the effects of electromagnetic interference, never use this unit in the EMG Mode, within 4 meters of a cellular telephone or near any other powerful radio interference-producing equipment that causes electrical sparks etc. In the EMG mode, this unit may be susceptible to strong interfering radio-type emissions that may lead to temporarily increased EMG microvolt readings. The reading will immediately return to the correct value when the interference ceases. (Remember that a relaxed muscle should read below 3.5 μVolts).
- Patient electrodes including all skin surface electrodes, vaginal and rectal probes are for single patient use only!
- Keep out of reach of children.
- Ensure that children do not inhale or swallow small parts.
- Do not use stimulation on your facial area unless you are under strict guidance from a qualified clinician.
- The application of electrodes near the thorax may increase the risk of cardiac fibrillation.
- Operation in close proximity (e.g. 1m) to shortwave or microwave therapy equipment may produce instability in the stimulator output.
- Simultaneous connection of a patient to high-frequency surgical equipment may result in burns at the site of the stimulator electrodes and possible damage to the stimulator.
- No modification of this equipment is allowed!
- The battery cover must be closed prior to the use of the device.
Contra-indications and precautions:
NMES (Neuromuscular Electrical Stimulation).
Neuromuscular Stimulation should not be used by:
- Patients fitted with demand-style cardiac pacemakers
- During pregnancy (unless medically advised)
- Patients with undiagnosed pain conditions
- Do not place electrodes:
– Over carotid sinus nerves
– Over larynx or trachea
– Inside mouth
– On anaesthetised or desensitised skin
– Do not drive a vehicle while the device is stimulating and attached to your body
– Do not apply stimulation across or through the head, directly on the eyes, covering the mouth, on the front of the neck (especially the carotid sinus) or via electrodes placed on the chest and upper back or crossing over the heart. - Skin irritation from the treatment of NMS or EMG itself does not generally occur. However, rubber electrodes may irritate some skin types, therefore, in this case we recommend to use hypoallergenic self adhesive electrodes.
- The patient should only use the unit for what it was prescribed for
- Do not immerse the unit in water or any other liquid substance
- Do not use stimulation on your facial area unless you are under strict guidance from a qualified clinician.
- Do not apply stimulation across or through the head, directly on the eyes, covering the mouth, on the front of the neck (especially the carotid sinus) or via electrodes placed on the chest and upper back or crossing over the heart.
EMG
There are no precautions when using EMG unless used for pelvic floor exercise or assessment. But EMG should not be used:
- During menstrual period
- If symptoms of bladder infection are present
- With patients who have diminished mental capacity or physical competence who cannot handle the device properly
Indications for Use:
NMES
• Stress urinary incontinence
• Overactive bladder (urge urinary incontinence)
TENS
• Pelvic Pain.
• Overactive bladder (urge urinary incontinence).
EMG
• Biofeedback for muscle rehabilitation after stroke and/or paresis
• Biofeedback for Pelvic Pain
• Stress urinary incontinence
• Overactive bladder (urge urinary incontinence)
• Faecal incontinence
ETS
• Muscle rehabilitation after stroke and/or paresis
• Stress urinary incontinence
• Overactive bladder (urge urinary incontinence)
• Faecal incontinence. -
Skin electrodes: round 30 mm

Reusable Long-term electrodes for TENS, NMS (STIM), EMG Biofeedback- Size: 30 mm dia – round
- Re-order code: VS30 – we don’t sell to general public, please obtain from your local distributor
- Recommended for: general use
- For single Patient use only!
Materials & Specifications
- Soft white cloth backing, suitable for most areas of the body
- High quality hydrogel and conductive carbon film
- Water based gel makes it skin friendly and allergy free (exclusions possible)
- Do not use alcohol wipes or other liquids to clean the front or gel side of the electrodes!
- Latex free
- CE marked
Skin electrodes: round 50 mm
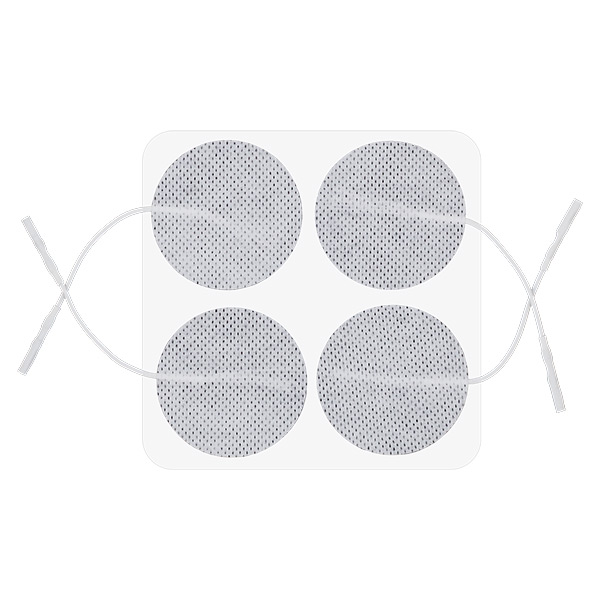
Reusable Long-term electrodes for TENS, NMS (STIM), EMG Biofeedback- Size: 50 mm dia – round
- Re-order code: VS50 – we don’t sell to general public, please obtain from your local distributor
- Recommended for: general use, as alternative to popular square shape of 50x50mm
- For single Patient use only!
Materials & Specifications
- Soft white cloth backing, suitable for most areas of the body
- High quality hydrogel and conductive carbon film
- Water based gel makes it skin friendly and allergy free (exclusions possible)
- Do not use alcohol wipes or other liquids to clean the front or gel side of the electrodes!
- Latex free
- CE marked
Skin electrodes: 50 x 50 mm
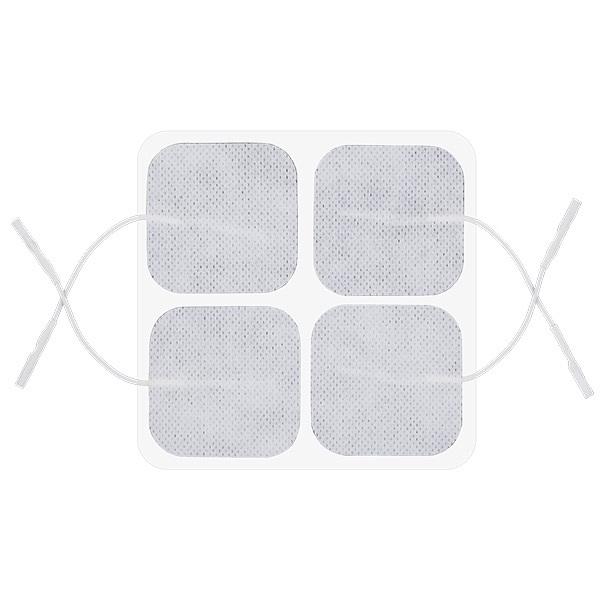
Reusable Long-term electrodes for TENS, NMS (STIM), EMG Biofeedback- Size: 50 x 50 mm – rectangular
- Re-order code: VS5050 – we don’t sell to general public, please obtain from your local distributor
- Recommended for: general use, this size is the most popular
- For single Patient use only!
Materials & Specifications
- Soft white cloth backing, suitable for most areas of the body
- High quality hydrogel and conductive carbon film
- Water based gel makes it skin friendly and allergy free (exclusions possible)
- Do not use alcohol wipes or other liquids to clean the front or gel side of the electrodes!
- Latex free
- CE marked
Skin electrodes: 90 x 40 mm

Reusable Long-term electrodes for TENS, NMS (STIM), EMG Biofeedback- Size: 90 x 40 mm – rectangular
- Re-order code: VS9040 – we don’t sell to general public, please obtain from your local distributor
- Recommended for: general use, slightly bigger alternative for popular 50 x 50 mm size, good for larger muscles, for TENS you may feel less irritation when using bigger electrodes on the back
- For single Patient use only!
Materials & Specifications
- Soft white cloth backing, suitable for most areas of the body
- High quality hydrogel and conductive carbon film
- Water based gel makes it skin friendly and allergy free (exclusions possible)
- Do not use alcohol wipes or other liquids to clean the front or gel side of the electrodes!
- Latex free
- CE marked
Skin electrodes: 90 x 50 mm

Reusable Long-term electrodes for TENS, NMS (STIM), EMG Biofeedback- Size: 90 x 50 mm – rectangular
- Re-order code: VS9050 – we don’t sell to general public, please obtain from your local distributor
- Recommended for: large muscles or TENS placing on the lower back
- For single Patient use only!
Materials & Specifications
- Soft white cloth backing, suitable for most areas of the body
- High quality hydrogel and conductive carbon film
- Water based gel makes it skin friendly and allergy free (exclusions possible)
- Do not use alcohol wipes or other liquids to clean the front or gel side of the electrodes!
- Latex free
- CE marked
Skin electrodes: 100 x 50 mm

Reusable Long-term electrodes for TENS, NMS (STIM), EMG Biofeedback- Size: 100 x 50 mm – rectangular
- Re-order code: VS10050 – we don’t sell to general public, please obtain from your local distributor
- Recommended for: large muscles or TENS placing on the lower back
- For single Patient use only!
Materials & Specifications
- Soft white cloth backing, suitable for most areas of the body
- High quality hydrogel and conductive carbon film
- Water based gel makes it skin friendly and allergy free (exclusions possible)
- Do not use alcohol wipes or other liquids to clean the front or gel side of the electrodes!
- Latex free
- CE marked
Lead wire – dual conductor

Dual conductor lead wire for TENS, NMS (STIM), EMG Biofeedback- Re-order code: LW103 – we don’t sell to general public, please obtain from your local distributor
- cable length: 1.25 mm
- Improved features: thicker, more flexible cable jacket.
Materials & Specifications
- Inner wire – Copper tinsel wire
- Outer jacket and plugs – TPU
- Latex free
Lead wire - REF
Single conductor Reference lead wire for EMG Biofeedback
- Re-order code: LW102 - we don't sell to general public, please obtain from your local distributor
- cable length: 1.00 mm
- Improved features: thicker, more flexible cable jacket.
Materials & Specifications
- Inner wire - Copper tinsel wire
- Outer jacket and plugs - TPU
- Latex free